Team members from left to right: Sahil Patel, Qian Zhong, Daniel Kim, Audrey Lord, Liang Hao, Sangeeta Bhatia, Kristin Moffitt, Loza Tadesse, Mohanraja Kumar, Marissa McDonald, Shih-Ting (Christine) Wang, Sakshee Jadhav, Brenda Kashi. Not pictured: Lucia Castello Pedrero, Tarek Fadel, Heather Fleming, Jeon W. Kang
MIT Koch Institute
August 2, 2023
Pneumonia is the leading cause of death among children worldwide, accounting for 14% of deaths among those under age five. Although pneumonia can arise from either bacterial or viral infection, reliable methods of diagnosing young children are invasive and expensive, frequently putting timely diagnosis out of reach of patients in low- and middle-income countries. Furthermore, new, simpler detection tools will also benefit patients in rural, under-served areas within the United States and other more resourced countries, as well. Where available, the standard treatment for pneumonia is an antibiotic. However, if given to a patient with a viral infection, antibiotics will do nothing to treat the infection and may also contribute to the growing problem of antibiotic resistance. Meanwhile, an expansion of available medicines to treat viral lung infections creates opportunities to save lives of children who are correctly diagnosed at an early stage.
Aiming to address this issue, Sangeeta Bhatia and her group have received a grant from Open Philanthropy to develop a non-invasive, accessible platform to diagnose pediatric pneumonia in under-resourced areas. In 2022, the Bhatia Lab demonstrated that their nanosensor diagnostic could reliably and quickly measure different immune responses to viral and bacterial pneumonia in preclinical models.
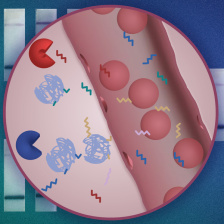
The sensor works similarly to a cancer diagnostic developed by Bhatia and her team over the past several years, where nanosensors carry synthetic biomarkers that are cleaved away when they encounter proteases (a type of enzyme) specific to the disease. For their pneumonia diagnostic, the Bhatia Lab used machine learning to identify the distinct protease signatures raised by the immune system’s response to bacterial vs. viral infections. Those signatures are detected in urine samples analyzed via a specialized, mass spectrometry test.
Bhatia, who is the John and Dorothy Wilson Professor of Health Sciences and Technology and Electrical Engineering and Computer Science, and a member of MIT’s Koch Institute for Integrative Cancer Research and the Institute for Medical Engineering and Science, will work closely with a team of local, leading experts including Dr. Loza Tadesse, the Brit and Alex d’Arbeloff Career Development Professor in Mechanical Engineering at MIT, Dr. Liang Hao, Assistant Professor in the Department of Biomedical Engineering at Boston University, and Dr. Kristin Moffitt, an Associate Physician in the Division of Infectious Diseases at Boston Children’s Hospital and Assistant Professor of Pediatrics at the Harvard Medical School.
The team will use the grant to adapt this pneumonia diagnostic for use in the doctor’s office and other points-of-care. Their plans include reformulating the nanosensors to be delivered with a nebulizer, similar to asthma inhalers, by repurposing tools that are already under development for use in administering cancer diagnostics. With colleagues from the Hao lab, the team will advance strategies to read out test results via simplified urine ‘paper’ lateral flow assays, akin to home COVID test kits. In parallel, the Tadesse lab partners will work on a complementary path to detect exhaled breath samples via optical point of care readouts. While the urine test takes only two hours and will be designed to be read with a simple cell phone photo, a “breathalyzer” approach could return results even sooner with the aid of a hand-held optical detection device. Finally, working with Dr. Moffitt and her team, the collective grant will also establish a biorepository of pediatric clinical pneumonia samples to use in cross-validation experiments that represent an essential early step ahead of progressing to human trials.
Re-engineering this diagnostic from a pre-clinical model in an academic setting into a ready-for-patients clinical platform requires significant effort and financing. Doing so with the help of a philanthropic investment enables an academic team to participate in the democratization of access to new, life-saving technology.