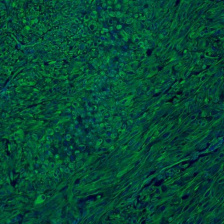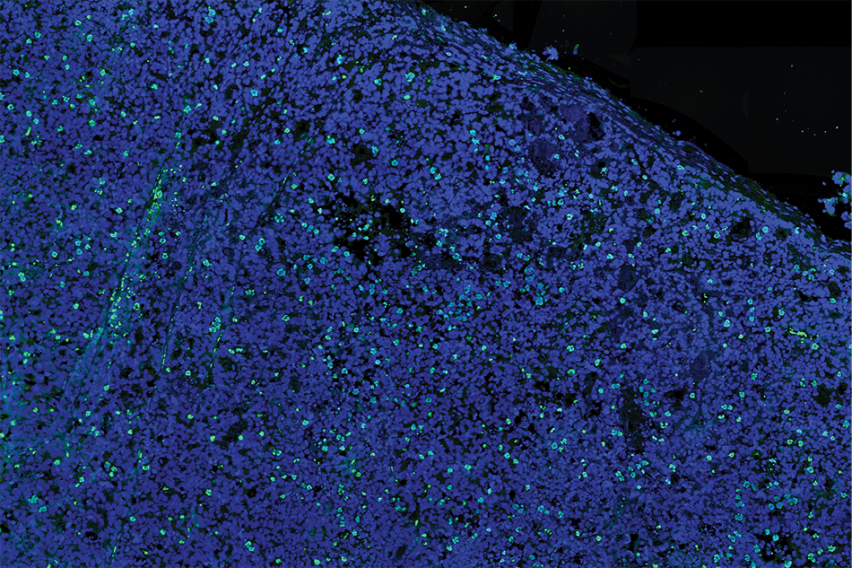
This Cancer Research cover image shows in vivo mitotic arrest and cancer cell death in tumor samples of castrate-resistant prostate cancer that has been treated with a combination of abiraterone and a Plk1 inhibitor. Nuclei are stained blue and mitotic cells are in green. There is an overabundance of cells in mitosis, the majority of which show signs of mitotic catastrophe.
MIT Koch Institute
January 31, 2023
The Yaffe Lab has discovered the mechanism responsible for the synergistic effects of a drug combination of Plk1 inhibitors, such as onvansertib, and abiraterone, a standard treatment for prostate cancer that works by reducing androgen synthesis and blocking the androgen receptor. In previous studies, the researchers identified this combination and found that abiraterone and Plk-1 inhibitors both interfere with cell division when delivered to cancer cells individually, yet when the drugs are delivered together, the effects are greatly amplified and led to far more cell death than either drug alone. Cardiff Oncology, the maker of onvansertib, is conducting clinical trials of the drug combination, with promising results in treating patients with metastatic castrate-resistant prostate cancer.
In a new study appearing in Cancer Research, the team discovered the biological mechanism behind the drug combination’s synergistic effects. Abiraterone by itself interfered with key steps in cell reproduction, namely mitotic spindle formation, chromosome condensation, and cell division. The researchers further found that when abiraterone is combined with a Plk-1 inhibitor, cancer cells undergo programmed cell death during mitosis, specifically at the spindle assembly checkpoint. Surprisingly, the researchers found that the drug combination’s effects are completely independent of androgen receptor signaling, and it was effective against a variety of other cancers, including some types of pancreatic and ovarian cancers and acute myeloid leukemia.
Synergistic combination therapies such as these, where the effects of the combo are more than additive, have the potential to be more effective than single therapies, preferentially target cancer cells rather than healthy cells, overcome resistance to treatment, and reduce toxic side-effects through allowing lower dosages of each agent. Since abiraterone is already approved by the FDA and the Plk1 inhibitor has performed well in clinical trials, the combination could be translated quickly into future clinical trials for cancer types beyond prostate cancer. This work was supported in part by the Bridge Project, a collaboration between the Koch Institute and Dana-Farber/Harvard Cancer Center.