
The Bridge Project grew out of conversations involving institutional leaders from the MIT and Dana Farber Cancer Institute communities, and key stakeholders including Art Gelb, shown here at a 2010 Bridge meeting with then-MIT President Susan Hockfield.
MIT Koch Institute
November 10, 2023
The Bridge Project: A Decade of Ongoing Impact
Launched in 2012, the Bridge Project embodies a simple idea uniquely suited to Greater Boston. Designed to leverage the collective expertise of MIT and Harvard, particularly its clinical cancer centers and teaching hospitals, the program’s goal is to enable collaborative research teams combining innovative science, tools and technologies with the translational expertise of clinical oncologists to solve challenging problems in cancer.
This ‘simple idea’ behind the Bridge Project was, in large part, the brainchild of the late Art Gelb, ScD ’61, who had close personal connections to cancer. Moreover, as an emeritus member of the MIT Corporation and emeritus trustee of Dana-Farber Cancer Institute, Gelb had unique insight into the workings of both institutions. Following extensive conversations with organizational leadership on both sides of the Charles River, a framework was born for how the Bridge Project might work. Gelb not only contributed to the vision for this effort, but also, through generous gifts with his wife Linda, provided initial resources to help realize and sustain the program, which runs entirely on philanthropy.
The program has now supported dozens of teams, who have developed significant advances in cancer detection, monitoring, and treatment, from completely new machine learning tools to novel devices that guide therapies, to better ways of using existing cancer medicines. From their Bridge Project roots, teams have gone on to pursue clinical trials and opportunities for direct clinical implementation, found companies to expedite translation, and win federal and private grants to sustain the work initiated via the Bridge Project.
Patients are always top of mind for the Bridge Project, yet this program has also altered the kinetics of research between and within member communities and, in several cases, the career trajectories of participating trainees and researchers—a new generation of cancer researchers who seamlessly engage the academic, clinical and industry sectors.
As the Bridge Project looks ahead, our focus remains on leveraging Greater Boston’s unique research landscape to seed collaborations that address the most challenging cancer problems and achieve translational goals that will make a real difference in the lives of real people.
Highlighted Projects
A rapidly clinically translatable, closed-loop drug delivery system to minimize pharmacokinetic variability of chemotherapies
2023 Traditional | Robert Langer and Giovanni Traverso, MIT’s Koch Institute; Douglas Rubinson, Dana Farber Cancer Institute
Chemotherapy dosing is based almost completely on a patient's body surface area or weight, which fails to account for many other sources of variability in pharmacokinetics—how the body absorbs, distributes, metabolizes, and excretes drugs—between patients. Factors such as sex, genetics and timing of administration can cause variability, resulting in considerable differences in a patient’s exposure to the active drug, and leading to suboptimal dosing, reduced efficacy and increased toxicity.
This team has developed a closed loop drug delivery system to enable physicians to control blood concentration of drugs in a patient regardless of altering factors. The system measures drug concentration via sensor, inputs these measurements into a control algorithm that adjusts the drug infusion rate to maintain concentrations at the desired level. The researchers have already demonstrated that the system can control a single common chemotherapy with widespread pharmacokinetic variability. In this project the team propose to improve the system to control the concentration of chemotherapies with longer half-lives, and of drug combinations, requiring control of concentrations of multiple chemotherapies simultaneously. Additionally, they will collect and analyze blood samples from cancer patients receiving the drugs tested in the system, to better understand correlations between concentrations and patient outcomes and determine how to program the closed loop system for clinical use. Upon translation, this system could provide broad benefit to cancer patients across tumor types, genetics, sex, chemotherapy regimens, and other factors, and improve the safe and effective use of many existing cancer medicines.
Translating AI for lung cancer risk into the clinic
2022 Traditional, 2020 Footbridge | Regina Barzilay, MIT’s Computer Science & Artificial Intelligence Lab and Koch Institute; Lecia Sequist, Massachusetts General Hospital
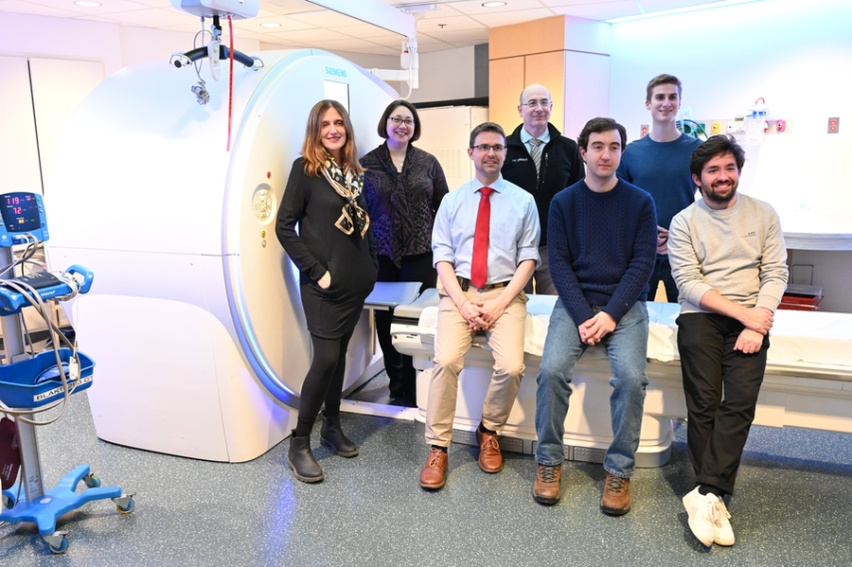
Barzilay (left), Sequist (second from left) and their team are developing new tools for lung cancer risk prediction and early detection.
Lung cancer screening methods and programs do not serve current needs, where an increasing proportion of lung cancer patients have never smoked or quit more than 15 years ago. This Bridge Project team is developing and testing Sybil, a new AI tool created in the Barzilay Lab to assesses a patient’s risk of lung cancer over six years by analyzing a single low-dose CT scan. Unlike current methods, Sybil can make accurate predictions without using demographic or medical information or a radiologist’s annotation. The tool could be used to identify individuals who need additional testing and closer management, and is particularly useful in addressing and this rising incidence of lung cancer among non-smokers and women. The team is using Sybil in conjunction with a lung cancer trial at MGH, with collaborating sites in Chicago, New York and Tennessee. Further, they are incorporating datasets from various demographic and occupational cohorts, to ensure that the tool will maintain its accuracy across diverse populations and reflect work-related exposures, such as those encountered by first responders, that can contribute to cancer risk.
Press: Sybil featured in STAT News, The Washington Post, MIT News; video presentation (Koch Institute)
Optimizing Plk1 therapeutics for clinical translation
Traditional | Steven Balk and David Einstein, Beth Israel Deaconess Medical Center; Michael Yaffe, MIT’s Koch Institute
Building on a previous Bridge Project collaboration in 2015 between the Yaffe, Balk and Jonas groups, this project also enlisted the participation of Cardiff Oncology, makers of the PLK1 inhibitor onvansertib, in launching a clinical trial to test a drug combination for advanced prostate cancer. The Yaffe Lab had previously found in pre-clinical studies a synergistic effect from combining PLK1 inhibitors with abiraterone, a drug used to treat advanced prostate cancer by targeting the androgen receptor to disrupt synthesis of the androgen hormone.
In this project, the team conducted studies to uncover the mechanism of action of this drug combination, and potentially biomarkers of treatment response, in conjunction with a clinical trial of the combination in patients with advanced prostate cancer. The researchers have made key discoveries about how the combination works, publishing earlier this year their surprising findings that the drug combination’s effects are completely independent of androgen receptor signaling, and it was effective against a variety of other cancers, including some pancreatic and ovarian cancers and acute myeloid leukemia.
Representing a previously unknown mechanism of action for this commonly used drug, the researchers found that abiraterone by itself interfered with key steps in cell reproduction, namely mitotic spindle formation, chromosome condensation, and cell division. Further, when abiraterone is combined with a Plk-1 inhibitor, cancer cells undergo programmed cell death during mitosis, specifically at the spindle assembly checkpoint. They have also identified a potential gene expression signature that could be used as a biomarker for predicting treatment response.
Data released from the clinical trial, which has progressed to follow on Phase II trials, the first of which are wrapping up this fall, showed that adding a Plk-1 inhibitor to daily abiraterone treatment in patients showing initial resistance to abiraterone resulted in durable disease control, with a significant increase in disease control rate in patients receiving the inhibitor for more days within a treatment cycle.
Press: Mechanism of action or Trial patient profile (MIT News); video presentation (Koch Institute)
Developing next-generation neoantigen-targeting vaccines for treating patients with metastatic cancer
2018 Traditional | Patrick Ott, Dana Farber Cancer Institute; Bradley Pentelute, MIT’s Koch Institute; Catherine Wu, Dana Farber Cancer Institute
New strategies are needed to bring the benefits of cancer immunotherapy’s initial successes to more patients and more tumor types. The Wu Lab has developed computational approaches to identify the best tumor antigens for therapeutic vaccines. They have generated a process to create personalized cancer vaccines addressed to these proteins, which harbor tumor-specific mutations—key elements that make tumors visible to the immune system and serve as ideal therapeutic targets for directing a patient’s immune response. The Wu lab demonstrated the safety, feasibility, and immunogenicity of the first version of this personalized vaccine, called NeoVax, in a proof-of-concept study of patients with high-risk melanoma.
This Bridge Project team formed to address the challenges of maximizing the tumor-targeting effect of the vaccine, and extending this approach broadly to many cancers. They have developed new machine learning tools to predict the optimal tumor antigens for vaccine use, and used a rapid protein synthesis platform and other technologies from the Pentelute Lab to help address the needs of high demand manufacturing and create novel delivery methods for maximizing the induction of anti-tumor immune responses. The team has been using new methods developed in the project in seven NeoVax clinical trials—in glioblastoma, renal and ovarian cancers, follicular lymphoma, chronic lymphocytic leukemia, and two in melanoma.
Moreover, data released from the initial melanoma trial indicates that these personalized cancer vaccines can create immunological memory. Trial data showed that four years after NeoVax treatment, all patients were alive—most with no signs of active disease—and analysis of patient T cells suggested the cells “trained” by the vaccine not only “remembered” their initial targets but had expanded their repertoire to recognize other melanoma-related ones as well.
Press: NeoVax trial (DFCI News)
Quantitative tumor oxygen measurements in cervical cancer patients
2017 Expansion | Michael J. Cima, MIT’s Koch Institute; Robert Cormack, Larissa Lee, and Ehud Schmidt, Brigham and Women's Hospital

As lead postdoc former Cima Lab member Gregory Ekchian played key roles in device design and trial. Now he heads Stratagen Bio, which he cofounded with Cima, a successful biotech entrepreneur, to bring these oxygen sensing radiation catheters back to the clinic for good.
Cancer patients undergoing targeted radiation therapy may soon benefit from technology developed in the Cima lab to address tumor hypoxia, a diminished supply of oxygen to malignant tissue that can be a serious barrier to effective treatment. Higher radiation exposure is required to kill cancerous cells in oxygen-depleted portions of a tumor, but physicians have not yet had practical ways of measuring oxygen levels within diseased tissue so that they can adjust dosage and precisely target this higher radiation in a timely way.
This Bridge Project team completed a first-in-human feasibility study in cervical cancer patients at Brigham and Women’s Hospital to develop and test the Cima Lab’s device, which incorporates tiny oxygen sensors into implantable radiation catheters to target high dose radiation therapy to specific areas of the tumor and kill resistant cancer cells. The device slots directly into existing clinical workflows and can even reduce the time patients normally spend in the MRI for dose planning.
Separately, the Cima Lab won additional funding via the Koch Institute Frontier Research Program to develop a second-generation oxygen sensing catheter that incorporates novel silicone materials and sensors developed by their group, as well as lessons learned from the Bridge clinical trial. The researchers have also founded a company, called Stratagen Bio, to bring these devices to the clinic.
The Bridge Project community and this team gratefully recognize the contributions of Dr. Larissa Lee, who passed away from cancer in 2021.
Press: feasibility study (MIT Spectrum); video presentation (starts at 2:54)
Targeting breast cancer cell metabolism to treat brain metastases
2017 Traditional, 2016 Footbridge | Rakesh Jain, Massachusetts General Hospital; Matthew Vander Heiden, MIT’s Koch Institute
Most breast cancer deaths result from complications after the tumor spreads, therefore, attacking the mechanisms underlying metastasis and therapeutic resistance is crucial. Treating brain metastases is especially challenging and therapy options are limited, particularly because cancer cells within brain metastases have access to different nutrients and respond differently to therapies that work to control disease elsewhere in the body. This Bridge Project team has been working to understand the mechanisms of resistance to HER2 targeted therapies in HER2-positive breast cancer brain metastases. At the collaboration’s launch, little was known about differences in metabolic requirements between primary and metastatic breast cancer, particularly in the brain. In their initial project the team found that the nutrients available to breast cancer cells in the brain differ from those available in the primary tumor. They also found that these differences create unique metabolic dependencies that might be exploited for therapy. One such difference, they found, has to do with the metabolism of fats. For breast cancer cells growing in the breast, fats are available in the tissue environment. Surprisingly, while there is fat in the brain, it is not readily accessible to the breast cancer cells, so they must make their own. Additional studies from this team have shown that a drug that stops the building of fats—already in early trials for patients with breast and other cancers—can increase tumor sensitivities to HER2 inhibitors used in the clinic and reduce breast tumor growth in the brain. Their findings have garnered interest from several pharmaceutical companies, and clinical trials to test agents that block fat synthesis in the brain are being considered to improve therapies for patients with brain metastases.
Press: study findings (MGH News)
Targeting minimal residual disease in acute leukemia
2015 Traditional | Scott Manalis, MIT’s Koch Institute; David Weinstock, Dana Farber Cancer Institute
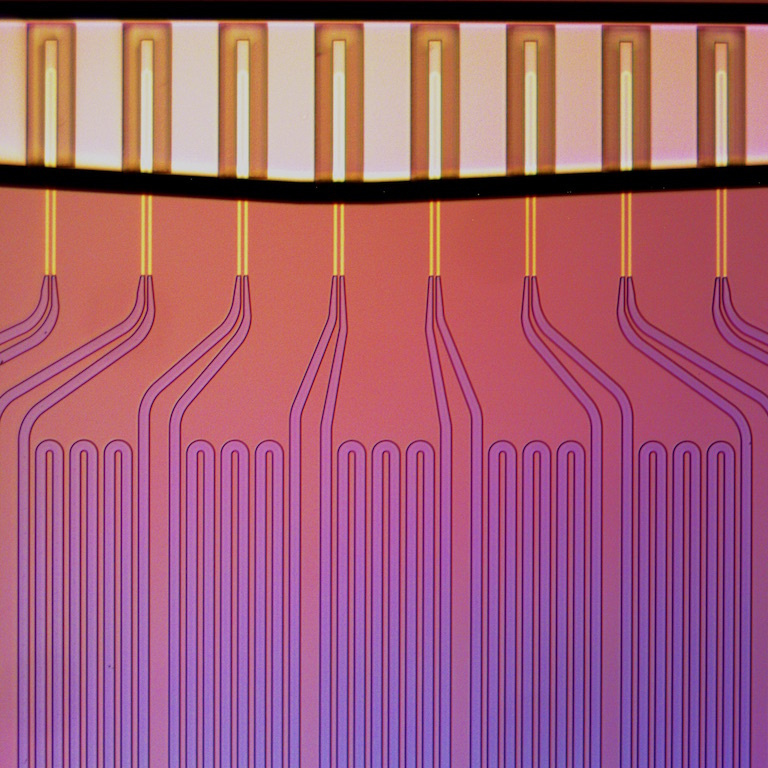
Microfluidics for the Masses: Measuring Cell Growth Rates, Koch Institute Image Awards winner 2017. As treated cells flow across the array of Manalis Lab sensors, each cell is weighed multiple times, thereby revealing the rate at which individual cells change their mass. Measurements of changes in tumor cell mass can be used to predict optimal treatment strategy for individual patients.
Predicting which medicines individual patients will respond to at a given stage of disease is essential to personalizing care and improving outcomes. Such predictions are particularly challenging in the many cancers where we lack biomarkers, or where disease progression is rapid or has already occurred, contexts where a nimble, device-based approach is especially useful. This Bridge Project team tested such an approach for clinical use in acute myelogenous leukemia, harnessing the Manalis Lab’s signature technology, the single microchannel resonator (SMR), a small microfluidic device that can be used to measure mass and other physical characteristics of single cells. The team has shown that changes in cell mass can accurately predict patient response to cancer therapies, and the SMR assay is currently in clinical studies in multiple liquid and solid cancers as a fast, inexpensive and non-invasive way to personalize patient treatment.
In a separate, follow-on clinical collaboration not supported by the Bridge Project, Dr. Manalis has worked with Keith Ligon at DFCI. The researchers tested the ability of the SMR assay to predict the response of individual patients with glioblastoma, an aggressive brain tumor, to temozolomide, a drug only effective in about half of patients. While there is a genetic biomarker for temozolomide, it does not always reveal drug sensitivity. The team showed that the SMR assay performed as accurately as the genetic biomarker, and moreover could be predictive in cases where that marker was not.
Drs. Manalis and Weinstock, along with Dr. Ligon—who has formally participated in the Bridge Project as a member of other teams—have founded a company called Travera, which is spearheading translation of this work.
Targeting mutant IDH1 in malignant gliomas
2013 Traditional | Daniel Cahill, Massachusetts General Hospital; William Kaelin, Jr., Dana Farber Cancer Institute; Matthew Vander Heiden, MIT’s Koch Institute

The late Steven Keating SM' 12, PhD'16 underwent the scanning technique developed by Bridge collaborators to monitor changes in 2HG levels. He holds a 3-D-printed copy of his brain tumor, which he printed using data from Brigham and Women’s Hospital.
IDH is a metabolic enzyme that when mutated produces an oncogenic metabolite called 2HG that accumulates to high levels in cancer cells, a discovery to which Dr. Vander Heiden’s work contributed significantly. In this Bridge Project team he and his collaborators worked to assess 2HG non-invasively in patients as a biomarker for brain cancers, and for use in imaging-based diagnostic approaches, helping to lay the groundwork for mutant IDH as a therapeutic target.
The team demonstrated that dynamic measurements of 2HG are feasible using 3D spectroscopy, and the decrease of 2HG levels can monitor treatment response in patients with IDH-mutant gliomas. Their results indicate that quantitative in vivo 2HG imaging may be employed for precision medicine and early response assessment in clinical trials of therapies that target IDH-mutant gliomas.
More recently, at the 2023 American Society for Clinical Oncology (ASCO) meeting, clinical researchers announced positive results from Phase 3 trials of a new therapy—which leverages research from Vander Heiden and this Bridge Project team—for treating low-grade IDH-mutant glioma, a type of brain cancer. The effectiveness of IDH inhibitor in low grade glioma is the first therapeutic breakthrough in this disease in more than 20 years, as early use of the drug significantly reduced the risk of disease progression or death. Beyond the direct promise of these clinical trial results for glioma patients, they also suggest potential new applications for the diagnostic techniques developed by this Bridge team and the research on which their work is based. Not only could the scan they developed be used to assess which patients might benefit from receiving the new drug, but also to monitor treatment response.
Press: Bridge screening and Steven Keating (includes video); 2HG Imaging (MIT News)

